Dr Grégory Guirimand

Établissement d'origine
École Supérieure des Sciences, de la Technologie et de l'Innovation, Université de Kobe - JP
Laboratoire d'accueil
Laboratoire Biomolécules et Biotechnologies Végétales (BBV), EA 2106, Université de Tours - FR
Hôte scientifique
Dr Vincent Courdavault
PROJET
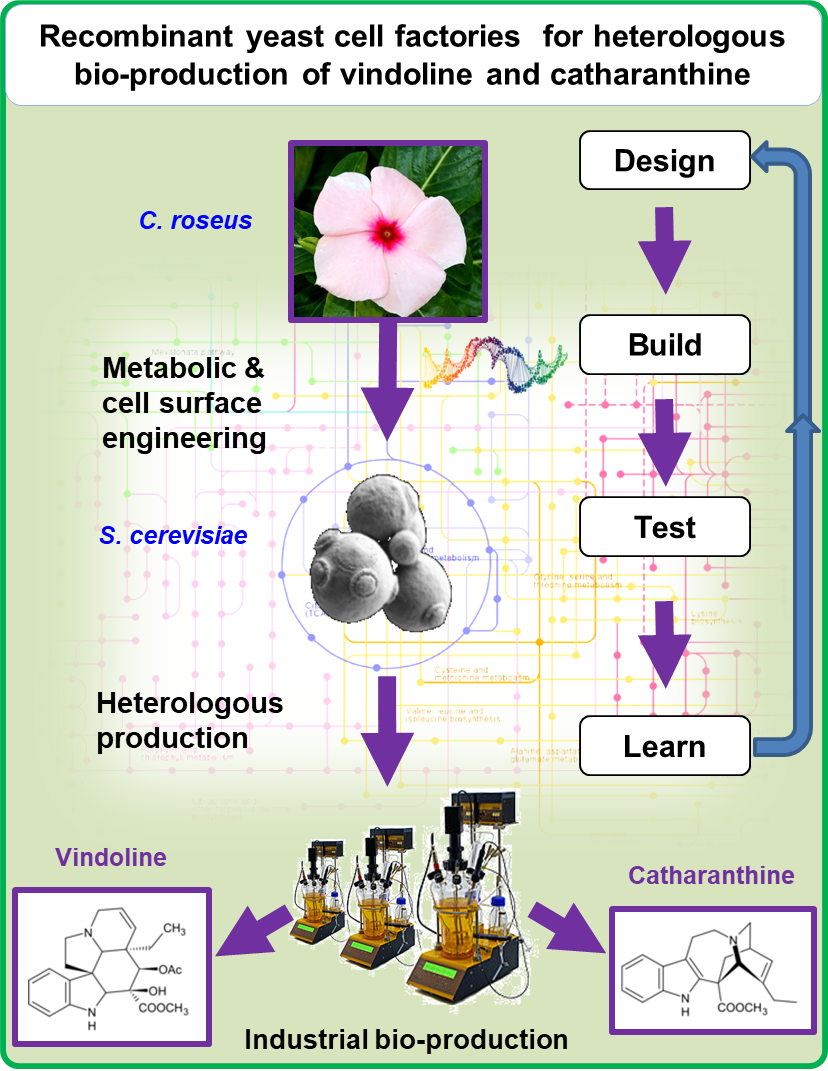
Bio-production of vindoline and catharanthine by recombinant yeast cell factories
The tropical plant Madagascar periwinkle (Catharanthus roseus) is naturally able to synthesize anti-cancer monoterpene indole alkaloids (MIA), such as vinblastine and vincristine.
The C. roseus MIA biosynthetic pathway is however regarded as the most complex pathway in all living organisms and shows surprising compartmentation at both cellular and subcellular levels. This drastic compartmentation is potentially responsible for the extremely low level of production of MIA in planta.
Our research project precisely aims to develop recombinant yeast cell factories in order to achieve heterologous bio-production of vindoline and catharanthine, two precursors of highly valuable anticancer MIA, originally produced by C. roseus.
To achieve this goal, a combination of metabolic and cell surface engineering approaches, using cutting-edge tools of synthetic biology in backer’s yeast (Saccharomyces cerevisiae) will be used.
Publications
The Madagascar periwinkle (Catharanthus roseus) synthesizes the highly valuable monoterpene indole alkaloids (MIAs) through a long metabolic route initiated by the 2C-methyl-D-erythritol 4-phosphate (MEP) pathway. In leaves, a complex compartmentation of the MIA biosynthetic pathway occurs at both the cellular and subcellular levels, notably for some gene products of the MEP pathway. To get a complete overview of the pathway organization, we cloned four genes encoding missing enzymes involved in the MEP pathway before conducting a systematic analysis of transcript distribution and protein subcellular localization. RNA in situ hybridization revealed that all MEP pathway genes were coordinately and mainly expressed in internal phloem-associated parenchyma of young leaves, reinforcing the role of this tissue in MIA biosynthesis. At the subcellular level, transient cell transformation and expression of fluorescent protein fusions showed that all MEP pathway enzymes were targeted to plastids. Surprisingly, two isoforms of 1-deoxy-D-xylulose 5-phosphate synthase and 1-deoxy-D-xylulose 5-phosphate reductoisomerase initially exhibited an artifactual aggregated pattern of localization due to high protein accumulation. Immunogold combined with transmission electron microscopy, transient transformations performed with a low amount of transforming DNA and fusion/deletion experiments established that both enzymes were rather diffuse in stroma and stromules of plastids as also observed for the last six enzymes of the pathway. Taken together, these results provide new insights into a potential role of stromules in enhancing MIA precursor exchange with other cell compartments to favor metabolic fluxes towards the MIA biosynthesis.
Final reports
The tropical plant Madagascar periwinkle (Catharanthus roseus) is a natural source of anticancer monoterpene indole alkaloids (MIA), such as vinblastine and vincristine, two molecules of major interest and therapeutic values. The MIA biosynthetic pathway in C. roseus is described in the literature as the most complex pathway in all living organisms and shows, in planta, an outstanding compartmentation at both cellular and subcellular levels. Our approach aimed to producing vindoline and catharanthine, two precursors of vinblastine and vincristine, in yeast cell factories. In particular, we developed and optimized yeast cell factories efficiently converting tabersonine to vindoline. First, fine-tuning of heterologous gene copies restrained side metabolites synthesis towards vindoline production. Tabersonine to vindoline bioconversion was further enhanced through a rational medium optimization (pH, composition) and a sequential feeding strategy. Finally, a vindoline titer of 266 mg/L (88% yield) was reached in an optimized fed-batch bioreactor. This precursor-directed synthesis of vindoline thus paves the way towards a future industrial bioproduction through the valorization of abundant tabersonine resources.


